Analyze the necessity of obtaining SFDA Saudi MDMA from the potential of the Saudi market
1. Market situation of Saudi Arabia. As one of the countries with the largest economic scale in the Middle East, Saudi Arabia has the second largest known oil reserves in the world. Its economic scale and per capita income level are among the best in the world, and it has an absolute position in the Middle East. dominant position.However, due to the underdeveloped domestic medical device industry, Saudi Arabia's medical devices are very dependent on the import market - mainly imported from the United States, the European Union, Canada and other countries.At the same time, Saudi Arabia’s competent authority SFDA also proposed the “Vision 2023”, striving to become an internationally leading science-based regulator to protect and promote public health.This also shows that Saudi Arabia is planning to expand its healthcare infrastructure and has continued to invest heavily in healthcare, which is a rare opportunity for domestic medical device companies.
2、 Introduction of regulatory authoritiesThe regulatory authority in Saudi Arabia is Saudi Food&Drug Authority (SFDA). Its main responsibility is to regulate, supervise, and monitor the safety of food, drugs, medical devices, cosmetics, feed, tobacco, pesticides, as well as to establish mandatory standard specifications.The main responsibilities of SFDA in the field of medical devices are:Establish regulations and procedures for the registration of medical devices and related products. Ensure the safety, quality, and efficiency of medical devices, laboratory materials and supplies, medical glasses, contact lenses, etc., and avoid their adverse effects on human health;Verify the safety and accuracy of calibration of medical devices and in vitro diagnostic reagents. Establish a comprehensive database for manufacturers and suppliers of medical device products;Establish necessary laboratories and physical testing facilities for testing the safety and effectiveness of medical devices;Monitor medical devices, laboratory materials, medical glasses, contact lenses, and other products during the sales process to ensure they are properly stored according to SFDA conditions;Track and monitor the operation of medical devices after their launch to ensure their safety performance;At the same time, ensure that medical device suppliers and distributors can fulfill their responsibilities in accordance with SFDA's conditions.
3. Saudi Arabia’s medical device regulatory framework
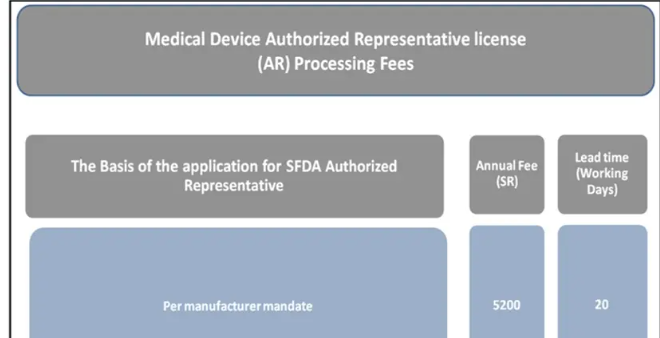
4、 Authorized Representative1. The conditions that authorized representatives should meet:Must be within Saudi territory; Must be within Saudi territory;Hold the AR license issued by SFDA for each manufacturer, and sign authorization agreements with manufacturers in accordance with current Saudi regulations;Meet the requirements of the quality management system;Record the implementation of necessary actions for executing delegated tasks and keep records of these tasks for SFDA inspection;Comply with SFDA requirements and publish relevant information on the SFDA website.2. Obligations of authorized representatives:The authorized representative shall represent the manufacturer in dealing with SFDA;The authorized representative shall cooperate with SFDA to carry out post listing supervision activities;The authorized representative shall notify SFDA of any events that occur outside the Kingdom of Saudi Arabia and have an impact on the circulation of medical devices within the Kingdom of Saudi Arabia. The authorized representative shall explain the situation and provide information on the corrective measures taken or proposed to be taken by the manufacturer;The authorized representative shall cooperate with personnel engaged in activities related to medical devices circulating in the Kingdom in accordance with the provisions of the Medical Device Law and its regulations. This should be recorded in the agreement between the authorized representative and the manufacturer;The responsibility of the authorized representative for the medical devices included in the agreement shall not terminate upon the request of the authorized representative to terminate the agreement, unless the manufacturer designates another authorized representative to replace it, or the medical device is no longer sold in the market;When it is necessary to terminate the agreement, the authorized representative shall notify the manufacturer and SFDA in writing;The authorized representative shall provide any information or relevant documents required by SFDA.3. Application fees and review time for authorized representatives:
5 . Classification rules for medical devices 1. Saudi Arabia’s classification rules for medical devices are similar to those of the EU MDR. Medical devices are divided into categories A, B, C, and D according to the risk level of the medical device from low to high.In addition, low-risk Class A instruments are divided into ClassA sterilization/ClassA measurement/ClassA reusable instruments.
The query method for Saudi Arabia's medical device classification is as follows: Query the classification of the device through "MDS-G008 Guidance on Medical Devices Classification"; In the SFDA's "Medical equipment list" database, search with the English name of the device to find similar devices in Classification by SFDA; Confirmation of device classification in Saudi Product Classification System (PCS) by a Saudi authorized representative.
6. SFDA’s requirements for quality systems: Medical device manufacturers must have a QM system that complies with ISO 13485:2016 standards.Ensure the safety and effectiveness of the device throughout its life cycle.
7. Medical device marketing path At present, all types of medical devices should follow the MDMA (Medical Device Marketing Authorization) certification path. Whether they are Class A low-risk or other high-risk devices, they should comply with the requirements of MDMA.Manufacturers outside Saudi Arabia should also appoint a local authorized representative to register MDMA on behalf of the manufacturer.The requirements for technical documents for MDMA certification are similar to those of the European Union. For details, please refer to Appendix 3 of "MDS–REQ 1 Requirements for Medical Devices Marketing Authorization".Note: According to Article 10 (18) of the "Implementing Regulation of the Law of Medical Devices", technical documents, labels and promotional materials should be provided in English. If the device is used by non-professionals, it needs to be provided in Arabic.
8、 Continuation and modification of medical devices1. Validity period and continuation method of certificatesAR license: This certificate is valid for one year and can be applied for renewal 60 days before the certificate expires. MDMA certificate: This certificate is valid for 3 years and can be renewed 90 days before its expiration. 2. Major changes to medical devices: Report to SFDA within 10 days after the change occurs, and provide the Medical Device Change Form and related documents on the GHAD system. Non significant changes: Report to SFDA within 30 days of the change and also report to MDMA.IT@sfda.gov.sa Send an email with the MDMA certificate number, which includes:Medical Device Change Form and related documents, orProvide a brief description of the changes (including device name, MDMA certificate number, medical device model, etc.), and then fill in the relevant change information within 90 days of the change.Note: The Medical Device Change Form and its definition of whether it is a significant change can be found in the MDS-G012 Guidance on MDMA Significant and Non Significant Changes.
Today's Arab International Medical Equipment Exhibition (Arab Health) is one of the largest medical equipment and healthcare exhibitions in the Middle East, held annually in Dubai, United Arab Emirates.The exhibition covers various medical fields, including medical equipment, medical equipment, hospital facilities, medical technology, medical information technology, medical consumables, pharmaceuticals, diagnostic equipment, rehabilitation equipment and services, etc. It attracts tens of thousands of relevant people in the medical industry to participate in the exhibition every year.
To support the SFDA Saudi market, we can assist domestic manufacturers with: Saudi medical device regulations related training; Saudi medical device product classification consultation; Saudi local authorized representative services; Saudi technical document writing services; Saudi MDMA declaration and registration services; Product testing services; Communicate with authorities and provide rectification services during the MDMA review process; provide technical services related to post-marketing supervision.



